SFB-Transregio 102
Polymere unter Zwangsbedingungen: eingeschränkte und kontrollierte molekulare Ordnung und Beweglichkeit
Der SFB Transregio 102 war ein langfristiges Grundlagen-Forschungsprojekt, das von der als Sprecherhochschule fungierenden Martin-Luther-Universität Halle-Wittenberg gemeinsam mit der Universität Leipzig beantragt wurde und durchgeführt war.
Um mehr über den SFB TRR 102 zu erfahren, besuchen Sie bitte unsere Archiv-Website.
Der SFB-TRR 102 war von der DFG gefördert.
1. Förderperiode: 01.07.2011 - 30.06.2015
2. Förderperiode: 01.07.2015 - 30.06.2019
3. Förderperiode: 01.07.2019 - 30.06.2023
Aktuelle Highlights aus der Forschung

Abschlussbericht des SFB TRR 102: Eine virtuelle Ausgabe unseres Sonderforschungsbereichs SFB TRR 102 "Polymere unter mehrfachen Zwängen: eingeschränkte und kontrollierte molekulare Ordnung und Mobilität" in Macromolecular Journals. 102 "Polymere unter mehrfachen Zwängen: eingeschränkte und kontrollierte molekulare Ordnung und Mobilität" in Macromolecular Journals.
Competition between crystal growth and intracrystalline chain diffusion determines the lamellar thickness in semicrystalline polymers
The characteristic morphological feature of semicrystalline polymers crystallized from the melt is a nanoscopic two-phase structure of thin lamellar crystals separated by disordered amorphous layers. This morphology is largely responsible for the favorable mechanical properties of semicrystalline polymers.
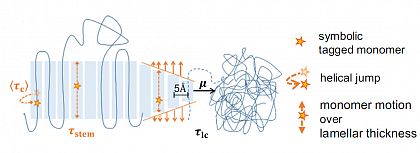
Our work addresses a classical question in polymer physics: which factors control the thickness of the crystalline layers? By combining a variety of complementary experimental techniques, especially small-angle X-ray scattering and NMR methods, we came to a consistent understanding that highlights the role of intracrystalline mobility, a typical feature of many semicrystalline polymers. The semicrystalline morphology is determined by the competition between crystal growth (characterized by the timescale of stem attachment) and simultaneous thickening of the crystallites (timescale of the intracrystalline chain diffusion as a measure for the mobility of the chains within the crystal). The results explain not only the strong temperature dependence of the crystal thickness found for some polymers but also the large variation of this quantity among different polymers affecting the degree of crystallinity and by that the mechanical properties of the material.
Hydrodynamic Manipulation of Nano-Objects by Optically Induced Thermo-Osmotic Flows
The manipulation, control and transport of molecules and particles in fluids is a key feature of microfluidics for all kinds of applications ranging from macromolecule analysis to chemical synthesis. Fluids in small channels are thereby driven by macroscopic pressure gradients, surface acoustic waves or electro-osmotic flows. We have developed a method that generates strong currents in small liquid volumes by remote heating of a thin metal film at a liquid-solid interface. The flows are created by van der Waals forces between water and the metal, which also facilitate nanoparticle trapping by interfacial forces. The dynamic variation of the temperature fields enables the generation of complex flow patterns that, together with other thermal effects also provides new ways to handle peptides in protein aggregation studies.
Chiral amines as initiators for ROP and their chiral induction on poly(2-aminoisobutyric acid) chains

Chirality is a unique characteristic of living systems, as an example represented as either left- or right-handed helical conformations with an outstanding impact to self-assemble into higher-order structures. α-Aminoisobutyric acid (Aib) is an achiral amino acids and known for its helical building properties, leading to either left or right-handed helices in poly(peptides). We have prepared poly(Aib)s by ring opening polymerization and investigated their preference to organize into either left- or right-handed helices by choice of a chiral inductor, placed at the head of the polymer chain. By using chiral amines as initiators for the polymerization reaction, we are able to induce a chirality on the poly(Aib) chain and can select a certain handedness helix by the choice of the initiator. As a major finding we could prove that chirality is induced only by the sterical influence of the initiator, preferentially via hydrogen bonds. The here investigated system thus allows to amplify chirality by only minor amounts of a chiral inductor, being potentially useful for spin-selection, chemical reactions or the organization of the helices into superstructures.
⇐ Benutzen Sie das Navigationsmenu links!
Die Abkürzung zu unserer Web-Seite:





